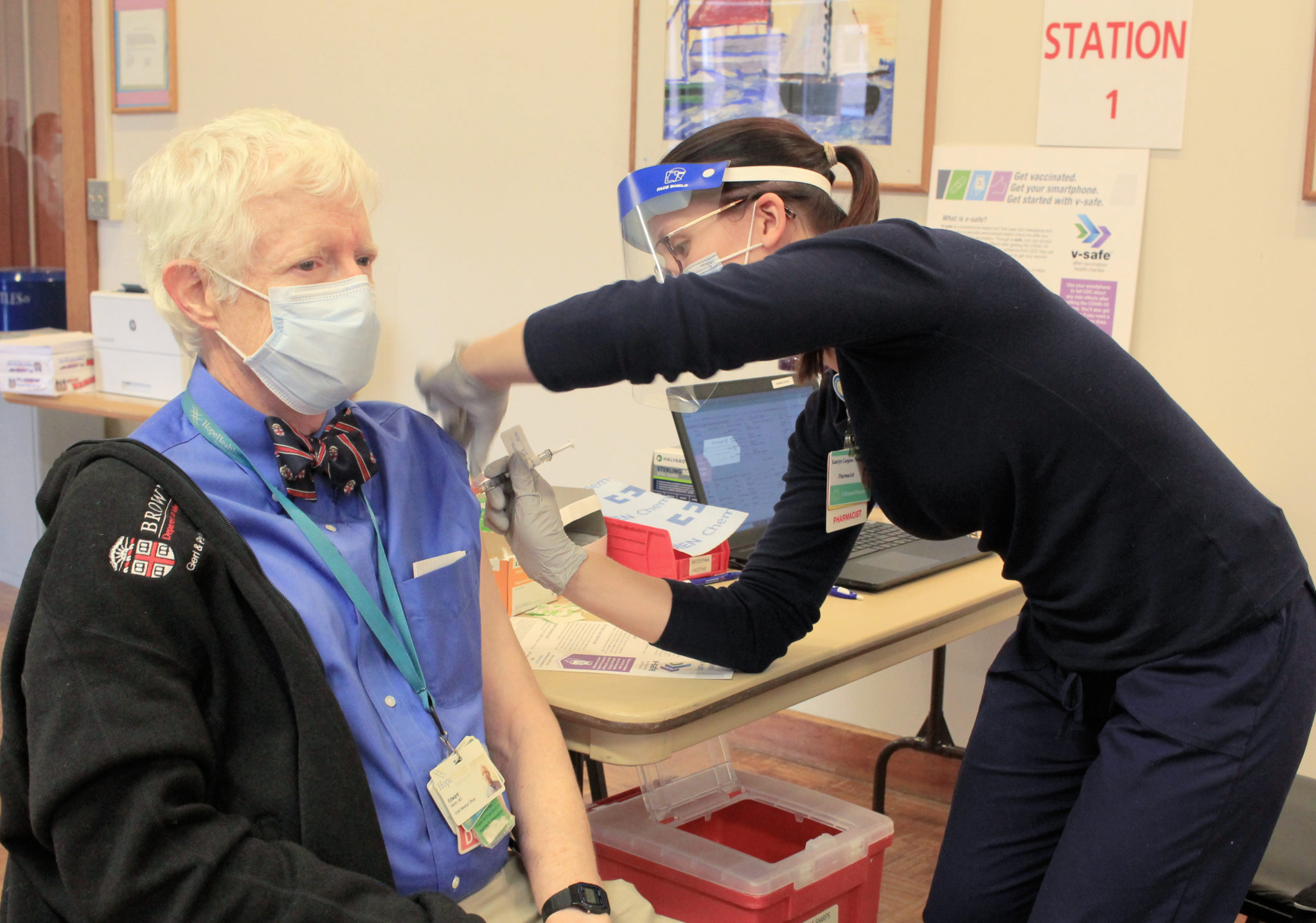
The two COVID-19 vaccines approved by the U.S. Food and Drug Administration in December can help end this pandemic. Inaccurate information shouldn’t be the reason you delay or decide against getting vaccinated.
MYTH 1: “The vaccine is unsafe because it was developed so quickly.”
FACTS: Work on the COVID-19 vaccine began right after scientists sequenced the genetic code of this coronavirus in January. But scientists had a head start.
That’s because they turned to a technology that has been around for over a decade called messenger ribonucleic acid or mRNA. Think of them as microscopic bicycle couriers delivering genetic instructions for our cells to make a harmless piece of spike protein found on the surface of the coronavirus. The messengers never enter the nucleus of the cell and are broken down after their job is done.
Traditional vaccines often require growing large amounts of a virus in eggs or cell culture over several months. Not so with mRNA vaccines, which aren’t made from live virus and can be made in a lab rather easily.
Using mRNA allowed the first phase of clinical studies to begin in March and the third phase trials in July without compromising on sound science and regulatory standards. The trials involved tens of thousands of people and took the same amount of time as other vaccine studies.
“Nothing about the way the vaccine was trialed and evaluated was rushed,” said Dr. Jennifer Ritzau, HopeHealth medical director and director of palliative care.
To save time, Ritzau added, the clinical trials happened at the same time as the preparation needed to begin large-scale manufacturing.
The federal government fast-tracked the review of efficacy and safety data by respected experts from around the country who recommended approval of two vaccines in December. A few weeks after receiving the second dose, you will be protected from COVID-19 at a success rate of about 95% if you received the Pfizer/BioNTech vaccine or 94.1% for the Moderna vaccine.
While no vaccine is 100% effective, the COVID-19 vaccines are among the very best efficacy seen with vaccines, Ritzau said.
“It’s astonishingly effective,” Ritzau added. “This a triumph of modern science.”
MYTH 2: “I’m young so I don’t need to get the vaccine.”
FACTS: Both younger and older people can get the COVID-19 virus.
And COVID-19 cases have been rising in children, adolescents and young adults since last summer.
Older people and those with pre-existing medical conditions like asthma, diabetes, and heart disease are at much higher risk of severe illness. COVID-19 has also led to serious illness and death in younger and middle-aged adults, even those who were otherwise healthy. Most children have mild or no symptoms, but some have gotten severely ill too.
Pfizer’s vaccine is authorized for ages 16 and up, Moderna’s for ages 18 and up. Both companies recently began new vaccine trials including children as young as age 12. If they’re successful, the data will need to go through FDA review.
MYTH 3: The vaccine is unsafe if you are pregnant.
FACTS: The FDA has left the decision of whether a pregnant woman should get the COVID-19 vaccine up to her and her health care provider.
“There’s not a lot of data yet on pregnancy,” says HopeHealth Medical Director Dr. Richard Long, a family physician who practiced and provided maternity care, newborn care, and high-risk obstetrics services for almost 30 years.
“But clearly a big concern would be women who have underlying medical conditions that would put them at particular risk of complications of a COVID-19 infection.”
Consider one study of U.S. women of childbearing age with COVID-19 during the first half of 2020. It found those who were pregnant were more likely to be hospitalized and at significantly higher risk for intensive care unit admissions and mechanical ventilation than those who were not pregnant.
Pregnant women—still generally excluded from research studies—were not included in the vaccine trials. But some women who received the COVID-19 vaccine during the trials became pregnant after vaccination and will be tracked to learn more about their experience. Moderna is establishing a “passive pregnancy registry” to monitor women who receive the COVID-19 vaccine.
Because of the lack of data, the World Health Organization (WHO) advises most pregnant women against getting vaccinated, except for those at high risk of exposure or having a severe case. Health care workers are among those considered at higher risk of unavoidable exposure so WHO recommends that pregnant health care workers consult their health care provider in deciding whether to get vaccinated.
It‘s important to remember that a number of studies show flu vaccines are safe for pregnant women. The American College of Obstetricians and Gynecologists (ACOG) recommends immunization against pertussis through a combination vaccine that includes tetanus and diphtheria, for pregnant women. ACOG also recommends that pregnant women who meet the criteria for receiving the COVID-19 vaccine should get it.

MYTH 4: Breastfeeding women should not get the COVID-19 vaccine
FACTS: Breastfeeding mothers were also excluded from Pfizer and Moderna’s vaccine trials.
The Academy of Breastfeeding Medicine says women who received the vaccine and are now nursing their babies should continue to do so. These mothers should discuss the risks and benefits of vaccination with their provider, especially weighing the potential for what contracting COVID-19 and developing severe illness would mean for their families.
The U.S. Centers for Disease Control and Prevention says mRNA vaccines are not thought to be a risk to the breastfeeding infant.
WHO also recommends lactating women should be offered COVID-19 vaccines and that it’s unlikely getting the shots poses a risk to breastfeeding children.
MYTH 5: The COVID-19 vaccine can make you infertile.
FACTS: Women who are thinking about getting pregnant or trying should know there is no evidence that vaccines affect their future fertility.
Because COVID-19 mRNA vaccines are not composed of live virus, they are not thought to cause an increased risk of infertility.
“There’s actually no data that suggests it causes infertility,” HopeHealth’s Dr. Long says. “I would struggle a bit to figure out the biological plausibility. It just doesn’t make sense.”
ACOG strongly recommends that vaccination for women actively trying to become pregnant or contemplating becoming pregnant. There is no need to delay or avoid pregnancy.
Women who conceive between the first and second dose of the vaccine should get their second dose of the vaccine at the appropriate interval.
The COVID-19 virus—not the vaccine—may impact sperm quality, which could contribute to problems with infertility for some men. Vaccination against COVID-19 may help to protect male fertility, however more research is needed.
MYTH 6: “I don’t have to get vaccinated because I already contracted COVID.”
FACTS: Immunity to seasonal coronaviruses is usually relatively short lived. That means reinfection with COVID-19 is still possible.
Immunity gained from having an infection—called “natural immunity”—varies from person to person. It is uncommon for people who get COVID-19 and recover from it to get it again within 90 days. Some studies have shown that people who recovered from a COVID-19 infection continue to produce protective antibodies for several months.
Beyond that, however, it remains unclear if these antibodies protect against infection and how long they last. We won’t know how long immunity produced by prior infection or vaccination lasts until there is more data.
For the time being, it’s hard to imagine life getting back to normal any time soon with more infectious strains of the coronavirus surfacing and the rollout of vaccines at a slower pace than many hoped.
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
Myth 7: I am already taking a lot of medications and don’t want the COVID-19 vaccine to mess with them.
FACTS: Approved vaccines generally shouldn’t have interactions with most medications people are taking, said HopeHealth Medical Director Jennifer Ritzau.
But the categories of people excluded from participating in the clinical trials for the two COVID-19 vaccines made for a very long list. These include people who are likely to be taking a variety of medications, such as those with diabetes and chronic pulmonary disease, including asthma, as well as chronic cardiovascular disease, HIV and autoimmune diseases like psoriasis and hypothyroidism.
“Immunosuppressants and those kinds of things are meds you should definitely talk to your doctor about,” Ritzau said.
Specialty physician groups and foundations focused on specific diseases have issued guidance statements. The National Psoriasis Foundation COVID-19 Task Force says patients with psoriatic disease who don’t have contraindications to vaccination should receive an mRNA-based COVID-19 vaccine as soon as it becomes available to them and continue their biologic or oral therapies.
People taking blood thinners can also be safely vaccinated if their condition is stable. The North American Thrombosis Forum says the COVID-19 vaccine is not associated with serious risk of bleeding but that bruising or very light bleeding can occur at the injection site.
The US. Centers for Disease Control and Prevention recommends not getting any other vaccine in the two weeks before or after you get your COVID-19 shots.
“It is out of an abundance of caution because you don’t want your immune system not to have a robust response to the virus,” Ritzau said.
“My advice is to prioritize this COVID-19 vaccine.”
Looking for more information about COVID-19? Visit our COVID-19 blog category.
SOURCES:
“Considerations for COVID-19 Vaccination in Lactation,” The Academy of Breastfeeding Medicine, Statement, December 14, 2020.
“COVID-19 Task Force Guidance Statements,” National Psoriasis Foundation. Last updated January 25, 2021.
“COVID-19 Update,” North America Thrombosis Forum, December 22, 2020.
“Flu and Pregnant Women,” U.S. Centers for Disease Control and Prevention. Page last reviewed September 22, 2020
“The story of mRNA: How a once-dismissed idea became a leading technology in the Covid vaccine race,” Damian Garde, STAT, and Jonathan Saltzman, Boston Globe, November 10, 2020.
“The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know,” World Health Organization, January 26, 2021.
“Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19),” National Library of Medicine, ClinicalTrials.gov.
“Study Finds Nearly Everyone Who Recovers From COVID-19 Makes Coronavirus Antibodies,” Dr. Francis Collins, NIH Director’s Blog, May 7, 2020.
“Vaccinating Pregnant and Lactating Patients Against COVID-19,” American College of Obstetricians and Gynecologists’ Immunization, Infectious Disease, and Public Health Preparedness Expert Work Group, Practice Advisory, December 2020.
“Why are mRNA vaccines so exciting?” Harvard Health Blog, Anthony Komaroff, MD, December 10, 2020
“Who can take the Pfizer-BioNTech COVID-19 vaccine?” World Health Organization, January 8, 2021.
“Understanding mRNA Vaccines,” U.S. Centers for Disease Control and Prevention, Updated December 18, 2020.